Newborn Screening Software Development Company
Table Of Content
Published Date :
13 May 2025
Build secure, compliant, and intelligent newborn screening software tailored for public health impact with Ditstek Innovations.
In newborn screening, every hour counts. We help state health departments, hospital networks, and diagnostic labs develop custom screening software that streamlines lab workflows, ensures timely intervention, and meets strict regulatory standards.
We provide:
- Tailored modules for specimen tracking, result management, and provider reporting and more.
- Seamless integration with LIMS, EHRs, and public health registries (HL7/FHIR-ready)
- HIPAA-compliant design with role-based access, full audit trails, and secure data exchange
Whether you're modernizing outdated infrastructure or launching a new screening initiative, our team brings deep healthcare expertise and a build-for-scale mindset.
Need a Custom Newborn Screening Solution?
We build secure, scalable software tailored for newborn screening programs. Let’s talk about your project.
What is Newborn Screening Software?
Newborn screening software is a custom digital solution that helps detect rare but serious health conditions in infants from the very first days of life. It helps healthcare providers, labs, and public health departments efficiently manage every step of the screening process, starting with specimen collection and lab processing and extending to reporting and follow-up tracking.
Unlike generic healthcare IT tools, custom newborn screening software is designed to fit the specific workflows and regulatory requirements of newborn screening programs. These systems are built to handle high volumes, reduce manual errors, and make sure that no critical diagnosis is overlooked or delayed.
At Ditstek Innovations, we work with decision-makers to create custom newborn screening software that’s built to meet clinical needs and public health reporting requirements.
Newborn Screening Software Market
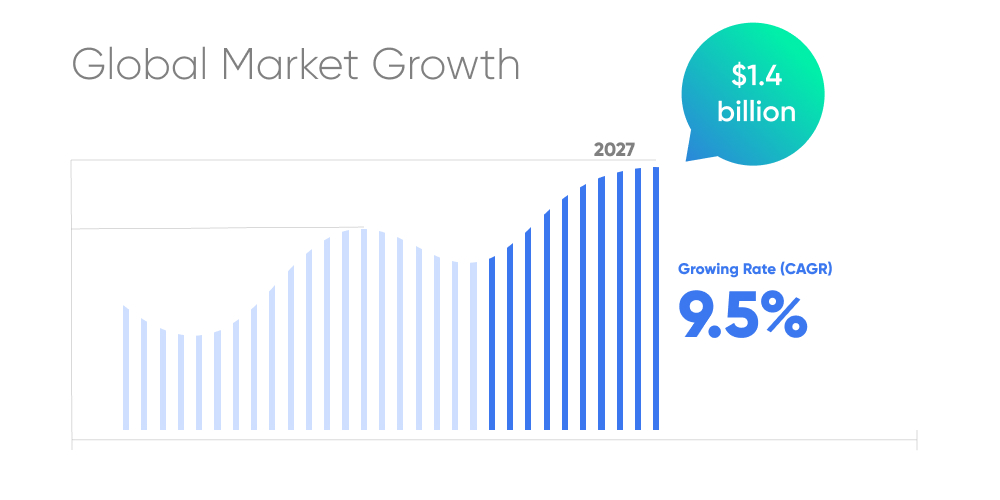
The global newborn screening software market is projected to reach $1.4 billion by 2027, growing at a compound annual growth rate (CAGR) of 9.5%.
Some of the key trends influencing the newborn screening software market are:
- The global expansion of screening programs, with countries increasing the number of conditions tested for at birth.
- The integration of AI in newborn screening software to enhance accuracy and decision-making.
- A shift towards interoperable systems that connect screening data seamlessly with EHRs, labs, and public health departments.
- Regulatory demands for enhanced data security, auditability, and real-time reporting.
Importance of Newborn Screening Software
Every year, over 13,000 infants in the U.S. alone are diagnosed with serious health conditions through newborn screening. These early screenings are often the only opportunity to detect disorders like metabolic, genetic, or endocrine before symptoms appear. But the system only works if it's fast, accurate, and coordinated.
Delays or errors in newborn screening can lead to missed diagnoses, lifelong health problems, or even loss of life. And when you're working with manual or outdated systems, the risk increases due to many factors like specimen mislabeling, data entry mistakes, and slow turnaround times.
Newborn screening software makes the process faster, reduces mistakes, and ensures results reach the right people on time.
Build Smarter Screening Systems Today!
Improve early detection, data accuracy, and patient outcomes with our specialized newborn screening software.
Newborn Screening Software Development Services We Offer
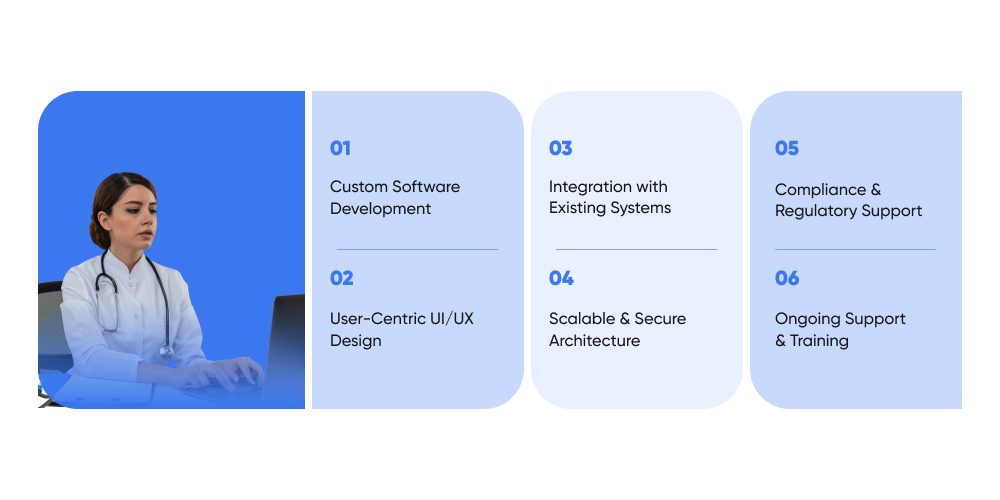
At Ditstek Innovations, we create newborn screening software that makes the process easier, more accurate, and compliant with health regulations. We work with hospitals, labs, public health teams, and software vendors to build custom software solutions according to their exact needs.
1. Custom Software Development
We develop tailored newborn screening solutions to meet the unique needs of your organization. From lab management systems to public health tracking platforms, we ensure the software supports your entire screening ecosystem, from specimen collection to reporting and follow-ups.
2. User-Centric UI/UX Design
Our design experts craft custom user interfaces tailored to each role in the screening process, ensuring that lab technicians, clinicians, case managers, and administrators can work efficiently with minimal training. We prioritize intuitive, responsive designs to drive quick adoption and reduce errors.
3. Integration with Existing Systems
Seamlessly integrate with EHRs, LIMS, and other health IT systems. We ensure your newborn screening software works harmoniously with your existing infrastructure, facilitating smooth data exchange and eliminating manual errors in workflows.
4. Scalable & Secure Architecture
Our software is built on secure, scalable architecture that supports both cloud and on-premise deployments. With advanced encryption, role-based access control, and compliance with HIPAA and other regulations, your data is always protected.
5. Compliance & Regulatory Support
We help you navigate complex compliance requirements by designing software that supports the latest healthcare standards, including HL7, FHIR, and government health reporting frameworks. We ensure your system is always up-to-date with new regulations, ensuring continuous compliance and reporting accuracy.
6. Ongoing Support & Training
Beyond development, we provide post-deployment support to ensure your system continues to function smoothly. From training your team to implementing software updates and addressing new regulatory requirements, we are with you every step of the way.
Key Features We Build Into Your Newborn Screening Software
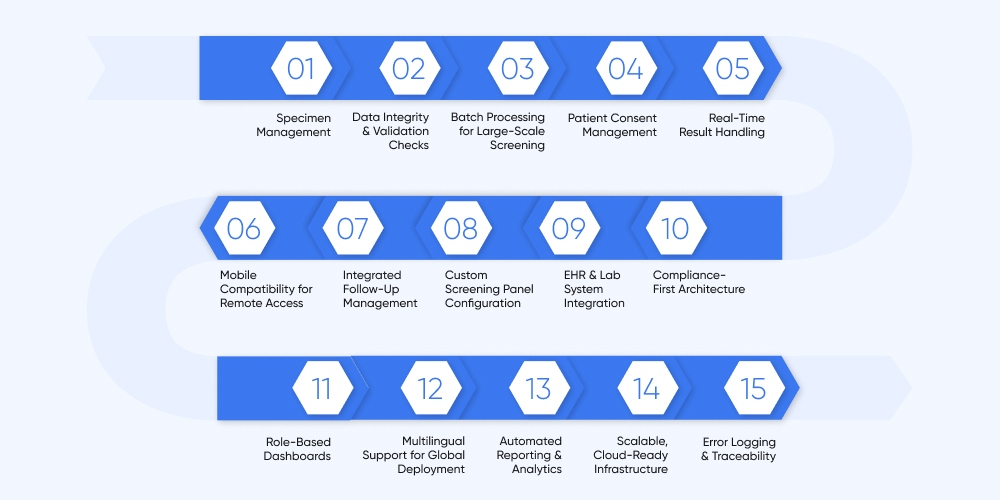
At DITS, we work closely with healthcare providers, labs, and public health agencies to build newborn screening systems that are secure, efficient, and tailored to your workflows. Here’s what we typically include:
Specimen Management
Track every sample in real time, from collection to lab to final diagnosis, ensuring traceability, faster turnaround, and accountability at every step.
Data Integrity & Validation Checks
Our system automatically detects data discrepancies and flags errors before they escalate, ensuring the integrity and accuracy of patient records and test results.
Batch Processing for Large-Scale Screening
For high-volume testing programs, our software supports batch processing to handle large datasets efficiently without compromising on speed or accuracy.
Patient Consent Management
Easily capture, manage, and track patient consent for screening and follow-up actions. This is essential for maintaining transparency and compliance with health regulations.
Real-Time Result Handling
Abnormal results trigger immediate alerts and follow-up workflows, helping care teams act faster and reduce time-to-treatment in critical cases.
Mobile Compatibility for Remote Access
Field staff, lab technicians, and clinicians can access the system securely on mobile devices, whether at home, in transit, or in remote areas, ensuring timely access to data.
Integrated Follow-Up Management
Manage re-tests, clinical referrals, and care coordination within one system. Every case stays visible, and no follow-up falls through the cracks.
Custom Screening Panel Configuration
Whether you're screening for 30 or 60+ conditions, our custom healthcare software solutions are tailored to your region’s guidelines, offering flexibility without compromising reliability.
EHR & Lab System Integration
We ensure seamless interoperability with EHRs, LIMS, and public health databases through HL7, FHIR, and custom APIs, reducing manual entry and duplication.
Compliance-First Architecture
From HIPAA and GDPR safeguards to role-based access and full audit logs, your system is built to meet today’s healthcare regulations and tomorrow’s inspections.
Role-Based Dashboards
We deliver customized dashboards for lab staff, clinicians, and program managers, ensuring the right data is available to the right people at the right time.
Multilingual Support for Global Deployment
Our solutions can be configured in multiple languages to accommodate diverse teams, ensuring effective communication and streamlined operations across different regions.
Automated Reporting & Analytics
Generate actionable reports for internal use or external agencies in just a few clicks. Track KPIs, turnaround times, and performance trends effortlessly.
Scalable, Cloud-Ready Infrastructure
Our solutions scale to meet your needs, whether you're managing a statewide program or piloting a new screening initiative, and are available in on-premise, cloud, or hybrid deployments.
Error Logging & Traceability
We provide comprehensive error logging features to track any discrepancies or system issues, with complete traceability, so your teams can review and resolve issues quickly and confidently.
Modernize Your Screening Infrastructure!
Say goodbye to outdated systems. Our team delivers modern, compliant, and scalable screening software.
Applications of Newborn Screening Software
From state-wide programs to hospital-based screening units, we develop custom solutions to support every stakeholder involved in protecting newborn health.
Public Health Screening Programs
Support large-scale screening initiatives with centralized control, standardized workflows, and real-time data visibility across labs, providers, and state agencies. Ensure faster follow-up and accurate case tracking.
Clinical Laboratories
Streamline specimen intake, automate result processing, and simplify reporting for thousands of samples per day. We build lab-focused modules that handle high volume without losing accuracy or compliance.
Hospitals & Birthing Centers
Enable seamless integration between bedside systems and lab workflows. Automate specimen collection tracking and ensure that screening orders, results, and follow-up tasks are fully connected to patient records.
Genetic & Metabolic Disorder Screening Units
Tailor your platform to cover specialized panels for rare disorders, carrier screening, or confirmatory testing. We develop software that handles both core and extended panels with equal precision.
Healthcare Networks
Unify multiple facilities under a single screening management platform with shared data access, flexible configurations, and role-based controls to standardize care across locations.
Research & Pilot Programs
Accelerate innovation with platforms that support experimental workflows, flexible data modeling, and high-integrity data capture, ideal for genomics, AI modeling, or early detection pilots.
NGO & Nonprofit Screening Initiatives
Support organizations working in underserved regions or global health programs. Our solutions can be scaled to fit low-resource settings while maintaining data integrity and offline capabilities.
Third-Party Screening Labs & Vendors
If you're a service provider offering screening for multiple hospitals or states, we enable multi-client workflows, flexible reporting formats, and secure client-specific data access.
Government & Health Agencies
Equip national or regional agencies with macro-level data, performance analytics, and reporting capabilities for compliance oversight and program evaluation.
Integration & Interoperability of Newborn Screening Software
We design custom solutions to fit into your existing health IT ecosystem, not disrupt it. We ensure smooth, standards-based data exchange with all critical platforms involved in screening and follow-up workflows.
HL7, FHIR & Other Interoperability Standards
We support industry-standard protocols like HL7 v2, HL7 FHIR, and CDA to ensure accurate, secure, and compliant data exchange across systems. Whether it's pushing lab results to an EHR or pulling patient demographics from a hospital system, the data flows where it’s needed without duplication or delay.
EHR, LIMS & LIS Compatibility
We help integrate software seamlessly with leading electronic health records (EHRs), laboratory information systems (LIS), and lab management tools (LIMS). This reduces manual entry, minimizes errors, and speeds up both specimen processing and care decisions.
Public Health & Government Portals
We’ve helped clients connect with state and federal systems for case reporting, birth registry matching, and public health analytics. Whether you’re submitting data to NBS programs or complying with national registries, we ensure that your system is ready and fully compliant.
API-First Architecture for Flexibility
Need custom integrations? Our API-first design enables secure, scalable connections with any third-party platform, giving you the flexibility to grow and adapt without major rework.
Ready to Transform Newborn Care with Tech?
Let us help you build innovative, secure, and accurate newborn screening solutions.
Our Newborn Screening Software Development Process
From first discovery to long-term support, we partner with public health programs, hospitals, labs, and vendors to build screening systems that are accurate, secure, and built to scale.
Here’s how we help:
1. Requirements Gathering & Workflow Mapping
We collaborate with stakeholders to define user journeys, compliance goals, and technical needs to ensure the software fits your exact program, not the other way around.
2. Role-Based UI/UX Design
Every user, including lab techs, data clerks, case managers, and physicians, gets a tailored interface built for speed and clarity. We prioritize usability to reduce errors, streamline data entry, and accelerate decision-making at every touchpoint.
3. Secure, Scalable Architecture
Whether you're deploying on cloud, on-premise, or hybrid infrastructure, we build systems that scale securely with your data and users. From data encryption and access control to audit logging and failover support, security is integrated into every layer of the system.
4. Seamless System Integration
We handle integration with hospital EMRs, lab equipment, LIMS, EHRs, and government health systems using HL7, FHIR, and other standards, ensuring your new platform connects smoothly with the ecosystem you're already running.
5. Post-Deployment Support & Training
After launch, we stay involved by resolving issues, training users, and helping your team stay current with evolving public health and compliance needs.
Why Ditstek Innovations for Newborn Screening Software Development?
Proven Healthtech Expertise
We've delivered digital health and public health solutions for over a decade, including screening systems, lab software, and regulatory-compliant platforms.
End-to-End Technical & Domain Strength
Our team combines HL7/FHIR experts, healthcare consultants, developers, and testers, so we understand both the workflows and the code.
Compliance-First, Agile Delivery
We incorporate security, privacy, and traceability from the start, while maintaining a flexible and iterative development approach.
Flexible Engagement & Long-Term Support
From MVP development to national-scale rollouts, we support different project scopes and stay involved post-launch with updates, integrations, and audits.
Frequently Asked Questions
1. What types of newborn screening programs can your software support?
We develop systems for a range of programs such as metabolic, genetic, hearing, and congenital screening, customized to your region’s requirements and workflows.
2. Can the software be integrated with our existing LIMS or EHR system?
Yes. We specialize in healthcare integrations, including HL7, FHIR, LOINC, and direct interoperability with EHRs, LIMS, state registries, and national databases.
3. How do you ensure compliance with health regulations like HIPAA or GDPR?
We design systems with compliance in mind using encryption, access controls, audit logs, and documented processes that align with HIPAA, GDPR, and local laws.
4. Do you offer on-premise and cloud-based deployment options?
Absolutely. We support both models based on your infrastructure, IT policies, and data residency needs.
5. Can the system be customized for different user roles like labs, hospitals, and public health agencies?
Yes. We design role-specific dashboards and workflows so each stakeholder, such as clinicians, lab techs, and coordinators, gets exactly what they need without the clutter.
6. How long does it take to develop a newborn screening system?
The development time totally depends on complexity, integrations, and compliance reviews. Most MVPs are delivered within 12 to 16 weeks, followed by continued iterations.
7. What post-launch support do you provide?
We offer flexible maintenance packages that include updates, training, technical support, and compliance-driven upgrades.
8. Can AI or decision support tools be included?
Yes, our services include integrating AI modules for anomaly detection, risk alerts, and data trends if your program is ready for predictive features.

Nidhi Thakur
With more than 19 years of experience - I represent a team of professionals that specializes in the healthcare and business and workflow automation domains. The team consists of experienced full-stack developers supported by senior system analysts who have developed multiple bespoke applications for Healthcare, Business Automation, Retail, IOT, Ed-tech domains for startups and Enterprise Level clients.
Recent Posts

Explore predictive maintenance software features that reduce downtime, cut costs, and boost efficiency with enterprise-grade, AI-powered maintenance systems.

Benefits of AI in real estate include better cash flow planning, improved marketing ROI, stronger pipeline visibility, and scalable growth.

We at DITS offer custom Population Health Management Software Solutions to help you measure the effectiveness and efficiency of care delivery to patients. Read our blog to know in detail.