Cost To Build Biotech Software Development
Table Of Content
Published Date :
16 Feb 2026
Key Takeaways
- Cost varies based on complexity, compliance, and integrations. MVPs require lower investment, while enterprise platforms demand higher budgets.
- Regulatory compliance significantly impacts cost and timelines. It must be planned from the beginning.
- System integrations and security architecture add substantial effort. These are often underestimated.
- Customization increases investment but improves long-term value.
- Phased development helps manage financial risk.
- Ongoing maintenance and compliance updates create additional lifecycle costs.
- When implemented correctly, biotech software delivers strong ROI through faster research and better compliance.
Overall, biotech software should be viewed as a strategic investment, not just a development expense.
Introduction
Biotech and healthcare organizations are under constant pressure to move faster without compromising compliance or data integrity. Research cycles are shrinking, regulatory scrutiny is tightening, and investors expect measurable progress. In this environment, digital platforms are the base of operational infrastructure.
Yet one question always surfaces in boardrooms: what is the cost to build biotech software, and is it justified?
The answer depends on scope, regulatory exposure, and long-term strategy. Some companies invest early and scale confidently. Others delay, then face fragmented systems, data silos, and expensive fixes. Understanding financial commitment upfront helps build systems aligned with business goals.
The right investment strengthens compliance posture, accelerates research, and improves decision-making across the enterprise.
Ready to Build a Compliant Biotech System?
Explore a structured development roadmap designed to balance validation, security architecture, and scalable automation from day one.
Key Factors That Influence Cost to Build Biotech Software
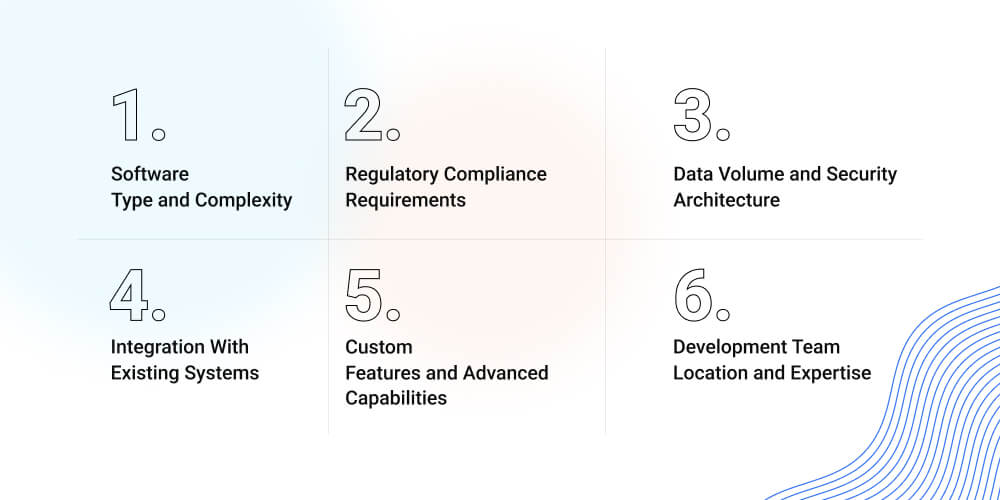
Every biotech platform is shaped by technical depth, integration scope, and security architecture. Understanding these variables early helps leadership teams forecast investment and costs accurately.
Software Type and Complexity
A lightweight research tracking tool differs vastly from an enterprise-wide LIMS integrated across multiple facilities. Scope drives budget.
Research-focused systems typically include:
- Experiment tracking
- Sample management
- Basic reporting dashboards
Enterprise platforms often require:
- Multi-site access controls
- Advanced analytics layers
- Integration with lab equipment and third-party APIs
- Custom workflows for R&D and quality teams
Data processing requirements also matter. Handling genomic datasets or multi-year clinical data archives increases infrastructure and architecture demands. Integration with sequencing machines or diagnostic instruments adds complexity.
In short, the broader the operational impact, the higher the investment. But so is the return.
Regulatory Compliance Requirements
Compliance is not an add-on feature. It shapes architecture from day one.
Common regulatory frameworks include:
- HIPAA
- FDA guidelines
- GxP standards
- GDPR
- 21 CFR Part 11
Meeting these standards requires:
- Detailed validation protocols
- Audit trails
- Secure electronic signatures
- Controlled document management
Compliance validation alone can add 15 to 30 percent to total project cost. Documentation, testing cycles, and external audits extend timelines. Skipping these safeguards is not an option. Regulatory penalties and product delays cost far more.
Data Volume and Security Architecture
Biotech firms handle sensitive patient records, proprietary formulas, and genomic sequences. A breach is more than an IT issue. It is a reputational crisis.
Security architecture typically includes:
- Role-based access control
- End-to-end encryption
- Intrusion monitoring
- Backup redundancy
Cloud infrastructure reduces upfront hardware investment but requires careful vendor compliance checks. On-premise environments offer tighter internal control yet demand higher capital expenditure.
The decision effects both operational expense and long-term scalability.
Integration With Existing Systems
Few organizations start from scratch. Most already operate:
- EHR or EMR platforms
- ERP systems
- CRM tools
- Laboratory devices and IoT-enabled instruments
Integration complexity influences development hours significantly. A disconnected ecosystem slows productivity. A unified environment streamlines reporting and compliance audits.
Many executives underestimate this phase. Integration alone can account for 20 to 40 percent of total development effort.
Custom Features and Advanced Capabilities
Modern biotech platforms increasingly include:
- AI-driven data analytics
- Automated research workflows
- Real-time dashboards for leadership
- Cross-team collaboration modules
Automation reduces manual errors in clinical documentation. Predictive analytics identifies anomalies early. And decision-makers gain live insights rather than waiting for quarterly reports.
When companies request tailored modules, budget scales accordingly. Customization always costs more than template-based systems. But it aligns the software directly with strategic advantage.
Development Team Location and Expertise
Location affects hourly rates. Expertise affects the quality of outcome.
Onshore teams may charge higher rates but often simplify communication. Offshore partners can optimize budget, provided domain expertise is strong.
What truly impacts financial planning is biotech domain knowledge. Developers unfamiliar with validation protocols or lab workflows require longer onboarding.
Partnering with an experienced biotech software development company like DITS reduces rework, shortens delivery cycles, and prevents expensive compliance oversights.
Estimated Cost Breakdown for Biotech Software Development
Below is a simplified cost overview based on typical project ranges.
| Software Level | Estimated Investment | Timeline | Suitable For |
| MVP Platform | $40,000 – $80,000 | 3–6 months | Early-stage biotech startups |
| Mid-Level Custom System | $80,000 – $200,000 | 6–10 months | Growing biotech firms |
| Enterprise-Grade Platform | $200,000 – $500,000+ | 10–18 months | Large pharma and healthcare enterprises |
MVP-Level Biotech Software
Focused on core functionality with limited integrations. Ideal for validating product-market fit. Many organizations leverage MVP development services to reduce early risk and attract funding.
Mid-Level Custom Biotech Platforms
Expanded compliance features, third-party integrations, and advanced dashboards. Suitable for scaling operations across departments.
Enterprise-Grade Systems
Full automation, predictive analytics, multi-location infrastructure, and regulatory-ready frameworks. Designed for complex ecosystems.
The cost to build biotech software increases with regulatory depth and integration scope. But phased planning can spread investment across milestones.
Planning Your Biotech Software Investment Strategy?
Get a detailed cost assessment aligned with compliance scope, integrations, and long-term scalability objectives for your biotech platform.
Timeline Vs Budget: How Development Phases Affect Cost
Typical development phases include:
- Discovery and requirement analysis
- UI and system architecture design
- Core development and testing
- Compliance validation
- Deployment and post-launch support
Discovery may last four to six weeks but prevents costly mid-project pivots. Development and testing often consume 50 percent of budget allocation. Compliance validation adds structured checkpoints.
Rushed timelines usually increase cost due to overtime resources and parallel QA cycles. Strategic pacing improves efficiency.
Cost Optimization Strategies for Biotech Businesses
Smart organizations manage investment without compromising quality.
- Begin with a structured MVP
- Plan compliance architecture from day one
- Choose scalable cloud infrastructure
- Engage domain specialists in biotech software development services
- Use agile delivery for phased releases
On top of that, technology partners can reduce long-term expenses by integrating automation and AI into workflows.
Need a Clear Budget Estimate for Your Platform?
Receive a tailored cost breakdown based on regulatory requirements, system complexity, and integration needs for your biotech initiative.
ROI: Is Biotech Software Worth Investment?
When implemented correctly, digital platforms deliver measurable impact:
- Research cycles reduced by 20 to 40 percent
- Documentation errors lowered significantly
- Faster audit readiness
- Improved cross-functional collaboration
- Long-term operational savings
One mid-sized biotech firm reduced compliance reporting time from three weeks to four days after system deployment. That efficiency alone justified the initial investment within 18 months.
Digital transformation in regulated industries is not experimental. It is strategic infrastructure.
Build Vs Buy: Which Option Makes Financial Sense?
Custom development offers:
- Full workflow control
- Scalable architecture
- Competitive differentiation
Off-the-shelf solutions provide:
- Faster deployment
- Lower upfront cost
- Limited customization
Hybrid approaches combine core packaged systems with custom integrations. For highly regulated research environments, customization often delivers better long-term alignment.
Organizations already investing in healthcare software development often benefit from extending internal platforms rather than introducing disconnected tools.
Why Choose DITS For Biotech Software Development
Selecting a partner influences outcome more than technology stack.
DITS delivers specialized biotech software development services designed for compliance-heavy environments. Our teams combine regulatory understanding with engineering depth to minimize rework and accelerate validation cycles.
At DITS, we use AI in software development, quality assurance, maintaining code quality, and deep customization. We integrate AI into every platform to enhance automation and analytics while maintaining strict compliance controls. Through targeted AI integration services, clients gain operational intelligence built directly into their systems.
The result is structured, scalable digital infrastructure aligned with research and regulatory goals.
Looking for a Trusted Biotech Technology Partner?
Connect with domain experts who combine regulatory understanding, AI-driven engineering, and scalable biotech platform architecture.
Conclusion
Executives often view digital platforms as expense lines. In biotech, they are growth catalysts.
Understanding the cost to build biotech software allows organizations to plan strategically rather than react defensively. Investment aligned with compliance, scalability, and automation transforms operations into a competitive advantage.
When software supports faster research, stronger compliance, and smarter decisions, it stops being a cost center. It becomes an asset that compounds value year after year.
Frequently Asked Questions
How Long Does It Take to Build Biotech Software?
Timelines depend on complexity, compliance scope, and integration requirements. A basic MVP can take 3 to 6 months, while enterprise-grade platforms with full validation and multi-system integration may require 10 to 18 months. Regulatory documentation and testing cycles often extend timelines, so realistic planning is essential from the start.
What Determines Cost to Build Biotech Software?
Several factors influence budget, including system complexity, compliance requirements, data security architecture, integrations with lab equipment or enterprise systems, and level of customization. The more advanced the workflows and regulatory controls, the higher the investment. However, phased development can help manage costs effectively.
What Is Average Cost To Build Biotech Software?
The cost to build biotech software typically ranges from $40,000 for a basic MVP to $500,000 or more for an enterprise-grade, compliance-heavy platform. Final investment depends on system complexity, regulatory scope, data security requirements, and third-party integrations. Companies handling sensitive clinical or genomic data should expect higher validation and infrastructure costs.
Are DITS Biotech Software Development Services Suitable For Startups?
Yes. DITS Biotech Software Development services are structured to support both early-stage biotech startups and large pharmaceutical enterprises. For startups, the focus is typically on building scalable MVP platforms that meet compliance standards while staying within budget. As the company grows, the platform can evolve without requiring a complete rebuild.
Can Existing Systems Be Integrated With New Biotech Platforms?
In most cases, yes. Modern architecture allows integration with EHR systems, ERP platforms, CRM tools, and laboratory instruments. However, the level of integration complexity will impact development time and cost. A detailed technical assessment is recommended before project initiation.
How Do DITS Biotech Software Development Services Ensure Compliance?
DITS Biotech Software Development services incorporate compliance planning from the discovery phase itself. This includes structured validation protocols, audit trail implementation, secure data handling mechanisms, and documentation aligned with regulatory standards such as HIPAA, FDA guidelines, and 21 CFR Part 11. Compliance is embedded into architecture rather than added later.

Nidhi Thakur
With more than 19 years of experience - I represent a team of professionals that specializes in the healthcare and business and workflow automation domains. The team consists of experienced full-stack developers supported by senior system analysts who have developed multiple bespoke applications for Healthcare, Business Automation, Retail, IOT, Ed-tech domains for startups and Enterprise Level clients.
Recent Posts

A practical development-focused guide on how to train an AI Agent using structured data, API integrations, testing, and workflow alignment for scalable business performance.
A practical guide explaining how to implement AI in business with responsible governance, secure integration, scalable architecture, and measurable operational impact across industries.

Explore how AI can be used in healthcare to improve diagnostics, automate operations, enhance revenue cycles, and deliver measurable performance outcomes across healthcare organizations.